QuestionQUESTION: We have lost 6 Koi ostensibly due to high PH. I have run some tests in our 200 gallon outdoor tank without fish and without chemicals, and I have found that the PH rose from 7.2 to over 8.2 within 3 hours after pump was started running. The filter was bypassed. Prior to running the pump the PH was stable in the 7.2 range over a 2-day period. The pump discharge was vertical downward into the tank from 10 inches above the surface. What do you think caused the PH to rise when the pump was running?
ANSWER: I would think that a couple of things could have happened... first would be that the pump stirred up some sediment that may effect pH... The second factor I would look at is temperature of the water. Warmer water holds less oxygen and oxygen can effect pH... pH is based on the free floating hydrogen ions which can combine with oxygen to form water... if the temperature changes and there is less oxygen then the pH will change too. pH is also effected by other water chemistry... did you check the water chemistry of the tank??? if so what was it??? dave
---------- FOLLOW-UP ----------
QUESTION: Thanks for the prompt response. It is greatly appreciated. We only have the following tests: Temp 77 deg F, Nitrate: negative, Nitrite: Negative, ammonia: negative, Hardness: soft,alkalinity:>300, PH:>8.4. The tank was purged with fresh water before the test. Very little sediment was in the tank. There could have been some sediment in the hose and the UV cartridge but I do not think so. I am wondering if it is excess oxygen from the high volume pump generating agitation and lots of bubbles on the surface. Being a Mechanical engineer and not a chemist, I understand physics better than chemistry.
AnswerHi John: On some level Physics and chemistry are very closely related... Most newly filled ponds have a higher pH then ponds that contain aged water because of the oxygen exchange at the surface as well as the time for the chemical reactions to occur and then dissipate. does the temperature of you pond remain constant??? or is there a fluctuation during the day and the night???? Have you tested the pH again to see if it has dropped???? dave

 koi health
Question
koi
hi one of my large chargoi has a la
koi health
Question
koi
hi one of my large chargoi has a la
 pH problems
QuestionQUESTION: Thank you for offering this service.
pH problems
QuestionQUESTION: Thank you for offering this service.
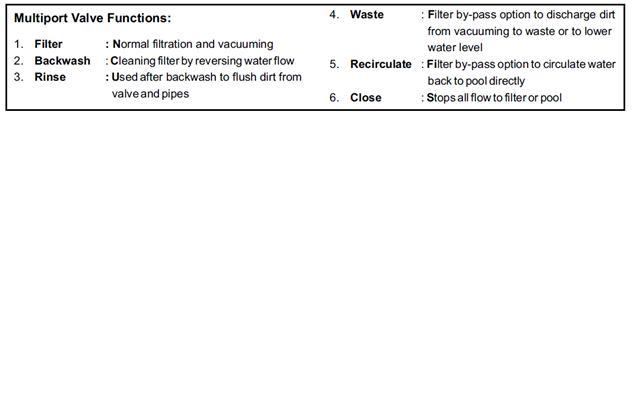 pond trouble
QuestionMe and my husband just bought a home with a pon
pond trouble
QuestionMe and my husband just bought a home with a pon
 over winter large koi in MN pond
Question
my pond
My pond is 4000 gallons, 4 feet deep a
over winter large koi in MN pond
Question
my pond
My pond is 4000 gallons, 4 feet deep a
 water pooling under liner/no hole in liner
QuestionQUESTION: I recently put in a small pond off of
water pooling under liner/no hole in liner
QuestionQUESTION: I recently put in a small pond off of